UV-C products present minimum risk when used by professionals who know how to use them. They must shield their eyes and skin to avoid light damage and severe injuries to the eyes and skin.
At this moment, none of our UV-C products are certified or approved under any applicable laws as a medical device and as such, Signify and/or any of its group companies do not currently intend for them to be used as medical devices anywhere in the world.
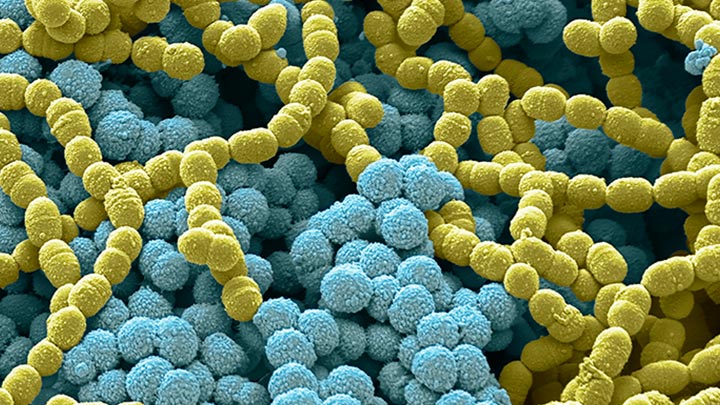
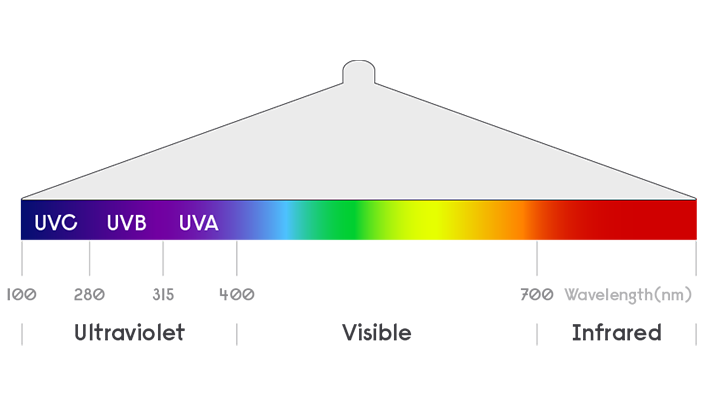
1) In laboratory testing, Signify’s UV-C light sources reduced SARS-CoV-2 virus infectivity on a surface to below detectable levels in as few as 9 seconds (Nadia Storm et al, Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation, 2020. Report available at https://www.nature.com/articles/s41598-020-79600-8). In this study, an exposure to an UV-C irradiance of 0.849 mW/cm2 for the duration of 9 seconds was applied, resulting in an UV-C dose of 7.64 mJ/cm2. Our UV-C surface disinfection products (fitted with our UV-C light sources) will achieve the same level of virus infectivity reduction as long as the same UV-C dose is achieved on each area of surface that is irradiated.
2) EPA Report, “Building Retrofits for Increased Protection Against Airborne Chemical and Biological Releases” Pg. 56
3) Fluence (UV Dose) Required to Achieve Incremental Log Inactivation of Bacteria, Protozoa, Viruses and Algae Revised, updated and expanded by Adel Haji Malayeri, Madjid Mohseni, Bill Cairns and James R. Bolton. With earlier contributions by Gabriel Chevrefils (2006) and Eric Caron (2006) With peer review by Benoit Barbeau, Harold Wright (1999) and Karl G. Linden
4) There is a limit to the amount of times certain objects (such as non-medical face masks) can be reused after disinfection with UV-C.